| Data request | |
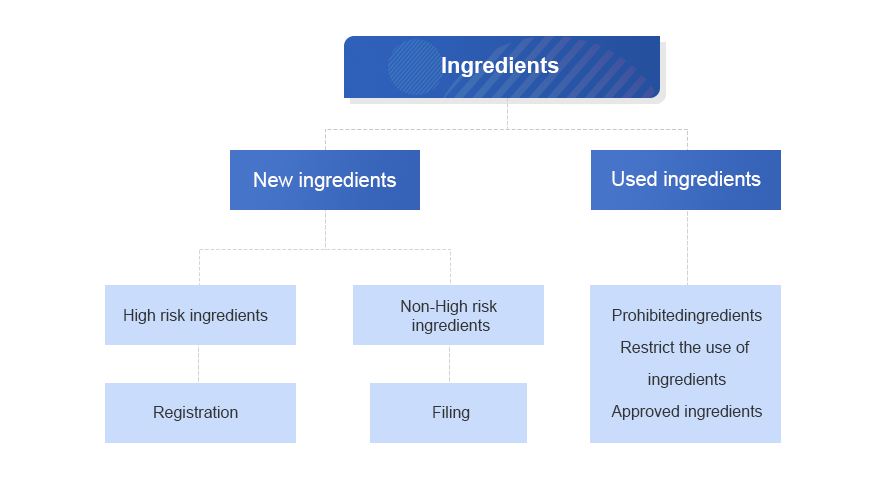
| 1.Basic information | 1. Registration or record ofingredients information form |
|
2. Research and development report |
2. Research and development background |
| 3. Basic information of ingredients | |
| 4.ingredients usage information① | |
| 5. Function basis data | |
| 6. Development of other related materials | |
|
3.The preparation process And quality control standards |
7. Brief description of preparation process |
| 8. Stability test data | |
| 9. Quality specifications and inspection methods | |
| 10. Information on possible safety risk substances and their control | |
| 4.Safety evaluation | 11.Overview of toxicological safety evaluation |
| 12. Acute oral or acute dermal toxicity test ③ | |
| 13. Skin and eye irritation/corrosion test | |
| 14. Skin allergy test | |
| 15. Skin phototoxicity test④ | |
| 16. Skin photoallergic test ⑤ | |
| 17. Mutagenicity test | |
| 18. Subchronic oral or dermal toxicity test | |
| 19. Teratogenic test | |
| 20. Chronic toxicity/carcinogenicity combined test | |
| 21. Inhalation toxicity test ⑥ | |
| 22. Long-term human trial safety test | |
| 23. Other toxicological tests⑦ | |
| 24. Safety assessment report | |
| 5. Other information | 25. Technical requirements for new ingredients |
| 26. Other materials that may help the registration and filing of new cosmetic ingredients⑦ | |